Drug development is costly and unpredictable, and value is often lost late, after major CMC and manufacturing investments are committed. Pharmatech Associates, a USP company, applies FDA aligned in silico CMC modeling to make development more predictable by de risking tech transfer and scale up, strengthening control strategies, and accelerating stability and shelf life decisions with scientifically justified, model supported evidence. By deepening process understanding and focusing experimentation where it matters most, programs can improve capital efficiency, compress timelines, and reduce CMC development spend by 60% or more in select cases through more efficient CDMO transfers and faster paths to market.
Using mature mechanistic, first principles models and deep regulatory experience, we enable risk based projections that can support filings ahead of long term data while strengthening Module 3 narratives and reducing CMC uncertainties that impact valuation in partnering, M&A, and exits. Delivered as Modeling as a Service, our infrastructure, governance, documentation, and FDA ready quality practices let you benefit without building internal modeling capability. With a strong track record supporting model informed FDA submissions, we help teams reach filing faster with lower risk, more reliable supply, protected investment, and stronger licensing value.


Every program is unique. Our modeling platform allows you to test and refine CMC strategies virtually, before committing resources. By simulating critical decisions and outcomes, we help you:
Whether your focus is out licensing, accelerated development, or scaling with limited resources, our team empowers you to make confident, data driven choices.
The FDA has encouraged the use of modeling and AI in drug development for nearly a decade, with hundreds of submissions referencing these tools. Pharmatech ensures your modeling strategy aligns with regulatory expectations to:
Case Example
A small biotech reduced its development timeline by more than 60 percent using Pharmatech modeling tools. In under eight months, the program advanced from design to tech transfer, gained pre IND alignment with the FDA, and entered Phase I manufacturing without pilot scale delays.

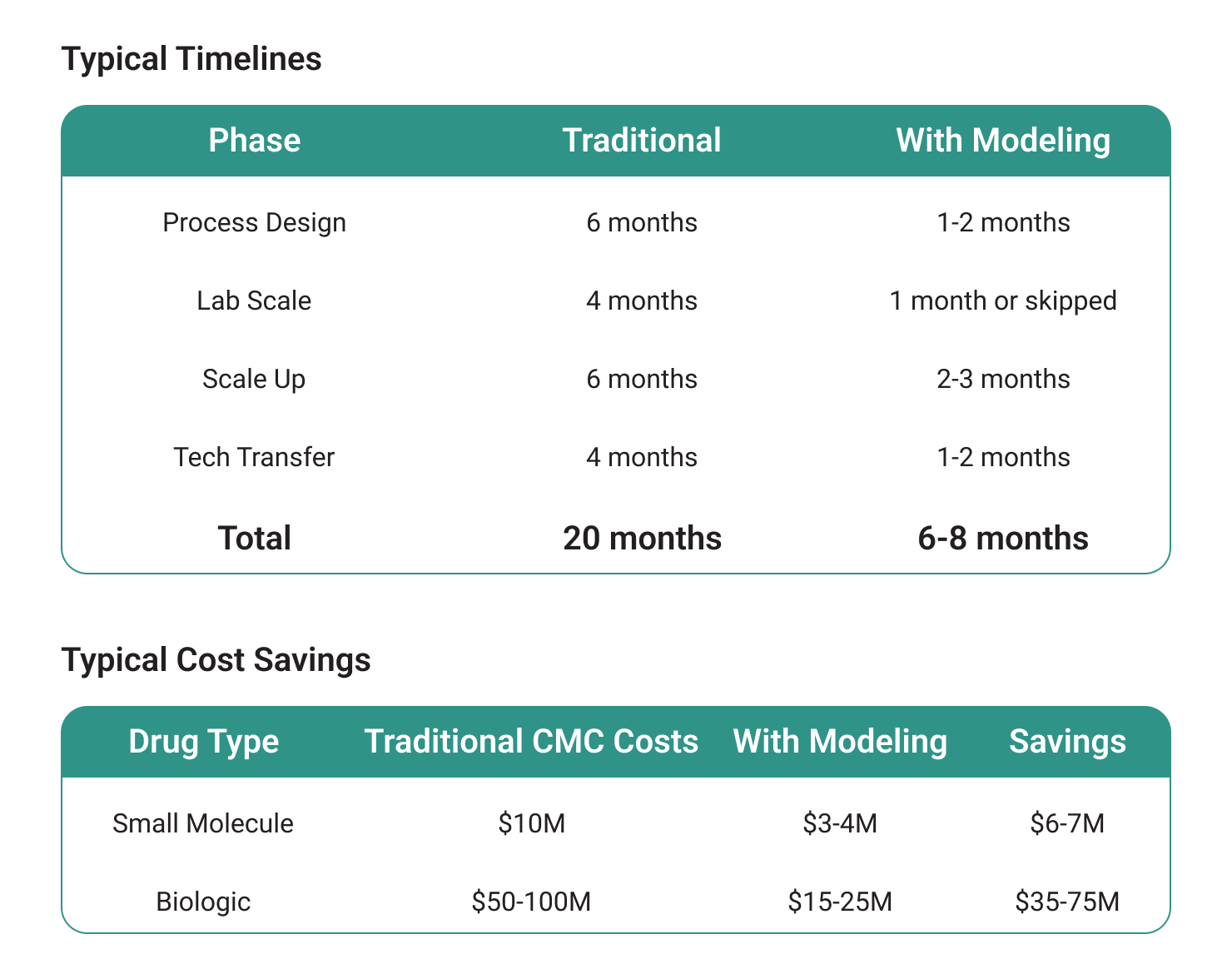
In silico modeling drives measurable efficiencies compared to traditional approaches.
Whether you are preparing an IND, evaluating out licensing, or scaling for commercialization, Pharmatech Associates provides the modeling expertise to reduce risk, shorten timelines, and unlock value.
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
Pharmatech Associates, Inc. 9110 Alcosta Blvd. STE H #601 San Ramon CA 94583